HIV Vaccine Reduces Infection Risk by 96%
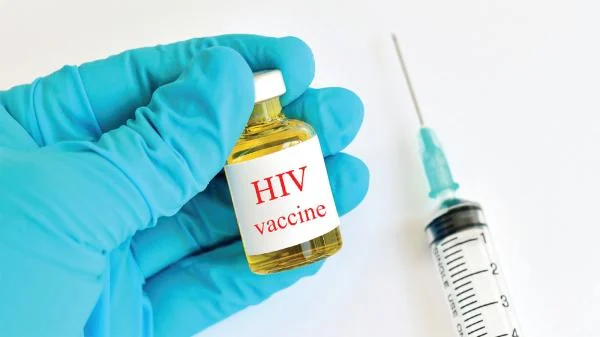
Gilead Sciences Inc announced that its semi-annual injection for HIV prevention has reduced the risk of infection by an impressive 96%.
The California-based biopharmaceutical company stated that its drug “Lenacapavir” has “proven superior” to “Truvada,” a daily pill used to combat HIV infection. Truvada was the primary option for pre-exposure prophylaxis (PrEP), which involves using medication to prevent the spread of the disease in individuals not yet exposed to the pathogen.
CEO Daniel O’Day said in a statement, “With these outstanding results across two phase 3 studies, Lenacapavir has demonstrated the potential to transform HIV prevention and help end the epidemic.”
A recent phase 3 clinical trial found that 99.9% of participants taking Lenacapavir for prevention did not contract HIV, with only two cases among a group of 2,180 participants, according to Gilead. In comparison, there were nine cases out of 1,087 individuals in the Truvada group.
Gilead noted that Lenacapavir was 89% more effective than Truvada.
The trial included male participants from various countries worldwide, including Argentina, Brazil, Mexico, Peru, South Africa, Thailand, and the United States.
Gilead reported no significant or new safety concerns from using Lenacapavir and Truvada.
These findings follow the results of a trial conducted in June, which found Lenacapavir to be 100% effective in preventing new HIV infections among women. There were no infections among the women and girls in South Africa and Uganda who received the injection.
O’Day stated that his company would work with regulatory, community, and governmental partners to ensure that “if approved, we can provide Lenacapavir twice a year for pre-exposure prevention worldwide, for all who want or need pre-exposure prevention.”
The data from both trials will be used to begin the drug approval process worldwide by the end of the year. Gilead stated that this might support the initial rollout of Lenacapavir by next year.
While the use of the injection has not been globally approved as a standalone prevention method, Lenacapavir has been approved by the FDA for treating HIV in adults, in combination with other medications.